 |
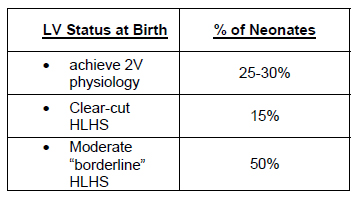
Fetal echocardiography provides the opportunity for the clinician to consider interventions aimed at changing the natural history of congenital heart disease at a time when changes occur rapidly. The relatively parallel circulatory arrangement of the fetal cardiac physiology further affords the opportunity to intervene on one side of the heart without necessarily compromising blood flow to the entire fetus. In addition, with improvements in echo resolution and increased experience with invasive procedures in the second and third trimester of pregnancy, in 2001 the Cardiovascular Program at Children's Hospital began a program to evaluate fetal interventions as a method of treating critical aortic stenosis, which results in hypoplastic left heart syndrome in the neonate. This program was based on observational studies which indicated that in a subset of patients, severe valvar aortic stenosis preceded left ventricular dysfunction followed by dilatation, and then growth arrest, resulting in an extremely hypertrophied, non-contractile left ventricle with a diminutive chamber. The working hypothesis was that the elevated afterload from valvar stenosis on the fetal LV caused dysfunction and dilatation with subsequent myocardial damage and growth arrest. The initial observations indicated that antegrade flow could be established in a significant proportion, and LV growth was observed in a subset of patients. At birth some infants had sufficient LV growth to permit two ventricle physiology, albeit with coarctation of the aorta and/or recurrent aortic stenosis in some. Results with fetal aortic valvotomy are shown in the Table.
 |
 |
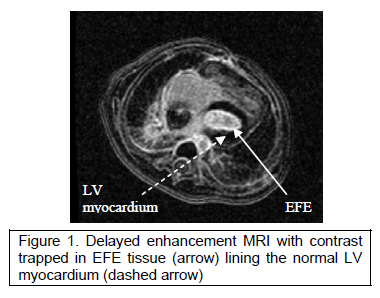
The neonates in this latter group have diffuse endocardial fibroelastosis with modest growth of the LV cavity pre-natally and no further growth post-natally. Intra-operative observation confirmed the echocardiographic findings of diffuse EFE and tethering of the papillary muscles creating a functional parachute mitral valve. Delayed enhancement MRI has been used to quantify the LV cavity volume and extent of EFE (Figure1).
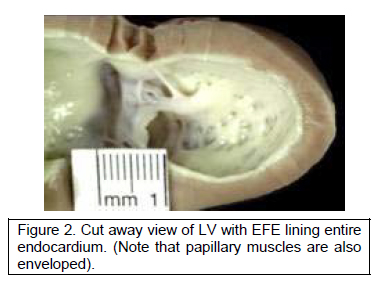
Our working hypothesis, modified to include our observations in post-natal hearts, then stated that the growth potential of the left ventricle was blunted by the presence of EFE. This was based on work by Taber indicating that ventricular growth is determined by end-diastolic wall stress imparted by fluid flow plus residual wall stress. The thick endocardial layer in EFE acts as a dampener of fluid-structure interaction, preventing LV growth (Figure 2).
The surgical protocol for the management of these patients with borderline left ventricles was been modified to include resection of endocardial tissue particularly in the posterior wall of the LV between the papillary muscles at the time of 1st Stage palliation; further resection of EFE and up-sizing of the systemic or RV to PA shunt with restrictive ASD at a subsequent stage. EFE resection, unlike removal of fibroelastic tissue from the sub-aortic area, requires sharp dissection since there is no cleavage plane between the EFE and myocardium. Resection of tissue from the interventricular septum and posterior wall, extending around papillary muscles to the LV apex can usually be accomplished through the mitral valve. The sub-aortic area can only be seen through the aorta, which also provides access to the dysplastic aortic valve for valvotomy.
Depending on LV growth, the ultimate goal is takedown of Stansel connection and systemic or RV to pulmonary shunt, resulting in a two ventricle repair. To date 15 patients have been included in this protocol with three patients completing all stages with resultant two-ventricle physiology.
References
- Armstrong EJ, Bischoff J. Heart Valve Development: Endothelial Cell Signaling and Differentiation. Circ Res. 2004;95:459-470.
- Bartman T, Hove J. Mechanics and Function in Heart Morphogenesis. Dev Dynam. 2005;233:373–381.
- Emery JL, Omens JH. Mechanical regulation of myocardial growth during volume-overload hypertrophy in the rat. Am. J. Physiol. Heart Circ. Physiol. 1997;273:H1198–204
- Salih C, McCarthy KP, Ho SY. The fibrous matrix of ventricular myocardium in hypoplastic left heart syndrome: a quantitative and qualitative analysis. Ann Thorac Surg. 2004 Jan;77(1):36-40.
- Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. Developmental Patterning of the Myocardium. The Anatomical Record. 2000; 258:319–337.
- Taber LA. Biomechanics of Cardiovascular Development. Annu. Rev. Biomed. Eng. 2001. 3:1–25.
|

